Introduction
Isolated extramedullary (IEM) manifestation of AML is a recurrent event especially after allogenic hematopoietic cell transplantation (alloHCT). To date, measurable residual disease (MRD) assessment for early detection and monitoring of this difficult-to-treat patient cohort has not been established. Using cell-free DNA (cfDNA) from plasma or serum for MRD measurement shows comparable sensitivity to bone marrow mononuclear cells (BMMCs) and improved sensitivity over peripheral blood mononuclear cells (PBMCs) in medullary AML (Nakamura et al., Blood, 2019). Here, we evaluate next-generation sequencing (NGS) for MRD assessment of cfDNA in a cohort of patients with IEM AML.
Methods
We retrospectively collected tumor tissue and matched BMMC/PBMCs and cfDNA serum or plasma samples from IEM AML patients at our institution. BMMC/PBMCs and IEM tumor tissue were sequenced with 35- to 48-gene panels at diagnosis and relapse. Amplicon-based NGS-MRD (sensitivity 0.01%) of matched cfDNA and BMMC/PBMCs was quantified at time of IEM manifestation and in all available samples collected up to 12 months before IEM manifestation using the known mutations from IEM AML tissue. Amplicon-based NGS and bioinformatic error-correction was conducted and analyzed as previously described (Thol et al., Blood, 2018).
Results
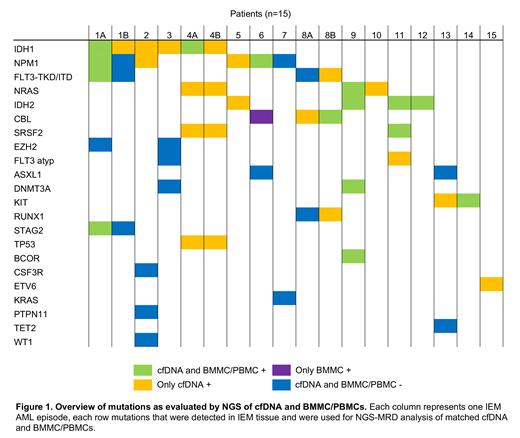
We identified a cohort of 15 IEM AML patients at our institution. One patient was diagnosed with IEM AML at first diagnosis, 14 patients suffered from IEM relapse. For three patients, matched BMMC/PBMCs and cfDNA samples were collected from a second IEM AML relapse during the disease course. 15 of 18 IEM AML episodes (83%) occurred as relapse after alloHCT.
42 AML-related mutations were identified in the IEM AML tissue with a median of three mutations per patient (range 1-5). 33 of 39 mutations (85%) found in IEM relapse tissue had been already present at first diagnosis. All mutations were then evaluated in cfDNA and BMMCs at the time of the IEM manifestation by sensitive NGS-MRD analysis for all 18 IEM AML episodes. For two IEM episodes, BMMCs were not available and PBMCs were used instead. 17 of 18 (94%) cfDNA samples were NGS-MRD positive at the time of IEM manifestation, while only 8 of 18 (44%) BMMC/PBMC samples were positive. 20 of 53 mutations (38%) at first and second IEM episode could only be detected using cfDNA, 15 mutations (28%) were detected in both cfDNA and BMMC/PBMCs and only one mutation was solely found in BMMCs. 17 mutations (32%) could not be detected by either method (Figure 1). The variant allele frequency (VAF) of mutations detected by both methods was significantly higher using cfDNA with a median VAF of 2.45% (range 0.12 - 24%) compared to BMMC/PBMCs with a median VAF of 0.048% (range 0.13 - 10%; p < 0.01). Median VAF of mutations that were only found in cfDNA was 0.63% (range 0.017 - 39.6) and did not significantly differ from the cfDNA VAF of mutations that were found by both methods (p=0.43), indicating that these mutations represented the major clone. However, no correlation was found between IEM tumor VAF and cfDNA VAF (r=0.01; p=0.95).
For ten IEM episodes from nine patients at least one cfDNA sample was available within 12 months before IEM relapse. In six IEM episodes (60%) cfDNA samples were MRD positive, one at 6 months, one at 5 months, two at 3 months and two at 2 months before IEM relapse. In four IEM episodes (40%) cfDNA samples were MRD negative, one at 10 months, one at 5 months and two at 2 months before IEM relapse.
Conclusion
In summary, cfDNA analysis from serum or plasma of AML patients can identify the molecular characteristics of a large proportion of patients with IEM AML. More than one third of clinically relevant mutations could only be detected using cfDNA and were missed by NGS-MRD of BMMC/PBMCs. Our data suggest that MRD monitoring from cfDNA detects IEM relapse earlier than routine monitoring from PB or BM in a significant proportion of patients.
Disclosures
Thol:Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees. Heuser:Agios: Research Funding; Certara: Honoraria; Pfizer: Consultancy, Honoraria; Novartis: Honoraria; PinotBio: Consultancy, Research Funding; Amgen: Consultancy; Loxo Oncology: Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Glycostem: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Astellas: Research Funding; BergenBio: Research Funding; Abbvie: Consultancy, Research Funding; Janssen: Honoraria; Servier: Consultancy; Sobi: Honoraria; Karyopharm: Research Funding; LabDelbert: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal